
“LIQVOR” is the first pharmaceutical company in Armenia after gaining independence.
It was established in January 1991 as a research and production enterprise.
On May 5 “LIQVOR” launched its first product and introduced 4 types of IV solutions.
“LIQVOR” reorganized its production areas and processes in accordance with the recommendations of the WHO GMP.

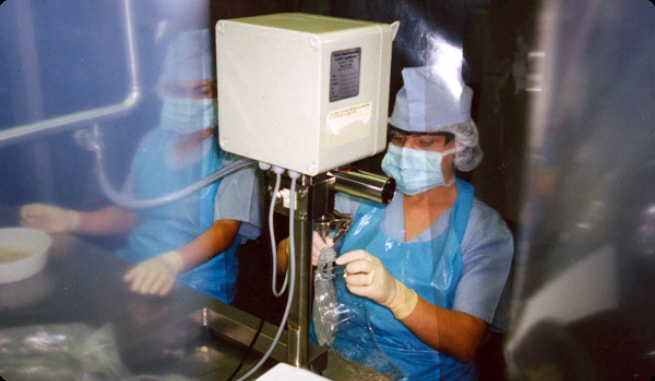
“LIQVOR” was the first pharmaceutical company in the post Soviet area to produce IV solutions in PVC bags.
Developed and introduced the production of IV antimicrobial fluoroquinolones in plastic packaging. In the same year the Company also launched the production of ophthalmic drugs.


“LIQVOR” has started to export its medicines to the Russian Federation and Republic of Georgia.
“LIQVOR” has acquired the French “Barriquard” and “Pierre Guerin” technological equipment which allowed to produce IV solutions exclusively in plastic bags.


“LIQVOR” was the first pharmaceutical company in the post Soviet area to develop and produce Moxicin® (moxifloxacin) IV drug.
In the same year, the director of the Company S. Matevosyan was awarded with the “Mercury” government award for the significant contribution to the development of the national economy.
LIQVOR signs an investment agreement with European Bank for Reconstruction and Development, for building a new plant in accordance with international standards. EBRD becomes a shareholder of the Company

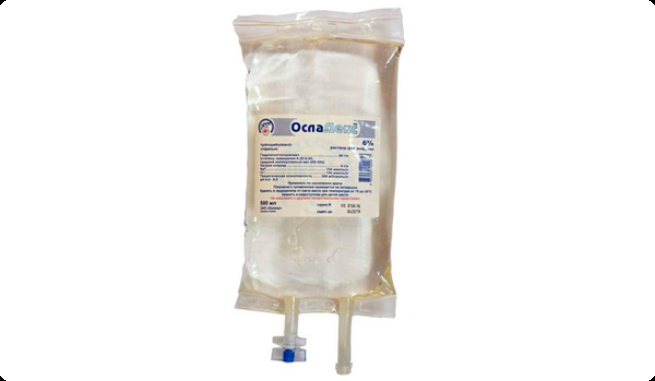
“LIQVOR”, the first in the CIS, introduces in production a new plasma-substitute preparation based on hydroxyethyl starch – OslaDex™.
The construction of the new plant started. In the same year, “LIQVOR” expanded its export geography by entering Belarus, Moldova, Kazakhstan, Uzbekistan and Tajikistan.


For the contribution to the development of the national economy, the Founding Director of the company S. Matevosyan was awarded with the Gold Medal of the Prime Minister of Armenia.
On the 20th anniversary of “LIQVOR”, a new plant in compliance with the requirements of GMP EU was started up.


“LIQVOR” was the first in the Caucasus to receive the Certificate of compliance with European standards of Good Manufacturing Practice (GMP EU) for sterile pharmaceuticals production. A joint audit was conducted under the supervision of an invited Head of the Israel GMP Inspectorate.
“LIQVOR” entered the Vietnamese market, which launched exports to South-East Asia and the Middle East.

The company was awarded with the “Pharmaceutical Company of the Year” award for its outstanding achievements in the pharmaceutical field. In the same year, the Company started exporting to the Republic of Yemen.
“LIQVOR” deserves a PIC/S GMP certificate by Ukrainian regulator and starts export to Ukraine.

Company started exporting to the Republic of Iraq.
“LIQVOR” started the establishment of its R&D center.
“LIQVOR” launched an official representative office in the Republic of Uzbekistan.
“LIQVOR” started celebration of its 30th anniversary with the launch of the new R&D center.


“LIQVOR” launched an official representative office in the Republic of MOLDOVA.